APPROVED FOR SINGLE-LEVEL TLIF
Fusion takes time.
ACCELERATE IT.

PearlMatrixTM P-15 Peptide Enhanced Bone Graft is the first and only bone growth accelerator shown to accelerate lumbar fusion in both the overall and higher-risk patient populations.*† Demonstrating more than twice as many patients fused at 6 months vs those using local autograft.1,2*
*
Demonstrated substantially faster time to fusion in a single-level TLIF PMA IDE study vs. local autograft.
†
High-risk includes nicotine use, BMI ≥ 30 and/or Type 2 diabetes.
It’s A Race Against Time.
†
High-risk includes nicotine use, BMI ≥ 30 and/or Type 2 diabetes.
PearlMatrix powered by P-15 Peptide is the first and only bone growth accelerator.1,2*
Accelerated fusion
Over twice as many patients fused at 6 months.1,2*
Over twice as many patients fused at 6 months.1,2*
- PearlMatrix was clinically proven to accelerate lumbar fusion in a Level 1, PMA, IDE study with ~60% high-risk patients.*†
- The significant difference in fusion speed between patients treated with PearlMatrix vs patients treated with local autograft demonstrated that PearlMatrix promotes statistically faster fusion vs local autograft*†
Substantially higher fusion rates at 6, 12 and 24 months in both the overall and higher-risk patient populations.1†‡
Substantially higher fusion rates at 6, 12 and 24 months in both the overall and higher-risk patient populations.1†‡
Evidenced by rigorous fusion assessment criteria using thin-cut CT evaluated by multiple independent reviewers.
Superior outcomes
Proven safety and efficacy with statistically superior overall clinical success at 2 years.1‡§
Proven safety and efficacy with statistically superior overall clinical success at 2 years.1‡§
- PearlMatrix demonstrated statistically superior overall clinical success at 24 months vs local autograft‡
- Overall clinical success was defined by a composite score of five components: fusion, function (ODI), neurological, no serious device-related adverse events and no index-level secondary surgical interventions
- To be considered an overall clinical success for an individual patient, all five components must be met
PearlMatrix was shown to be as safe as local autograft.1‡
PearlMatrix was shown to be as safe as local autograft.1‡
- No observation of ectopic bone formation‡
- No meaningful differences in reported seroma formation‡
- No incidents of osteolysis‡
- No meaningful differences in reported radiculopathy‡
Ease of use

PearlMatrix is formulated to optimize handling and efficiency in the operating room:1,2

PearlMatrix is formulated to optimize handling and efficiency in the operating room:1,2
- Conveniently packaged as a freeze-dried material that does not require freezing or thawing
- Quickly hydrated with sterile surgical solution (saline or Ringer’s lactate)
- Designed to stay where you put it with a moldable collagen matrix carrier designed for retention at the fusion site
See Important Safety Information
*
Demonstrated statistically superior time to fusion in a single-level TLIF study vs local autograft.
†
High-risk includes nicotine use, BMI ≥ 30 and/or Type 2 diabetes.
‡
As demonstrated in a single-level TLIF study vs local autograft.
§
Overall clinical success is defined by a composite score including fusion, function (ODI), neurological, no serious device related adverse events and no subsequent surgical interventions.
PearlMatrix was rigorously tested in the ASPIRE PMA study.
A 293-patient, pivotal, premarket approval (PMA) study vs local autograft, inclusive of ~60% high-risk patients.1†
A prospective, randomized, controlled, statistically-powered PMA study
- PearlMatrix vs local autograft
- Single-level TLIF (L2-S1) with any FDA-cleared static PEEK TLIF cage
Primary endpoint: 24-month composite clinical success (CCS)
- Five components: fusion, function (ODI), neurological, no serious device-related adverse events and no index-level secondary surgical interventions
- CT follow-up: 6, 12, and 24 months
Evaluated in a real-world patient population
- 293 patients
- 33 US clinical trial sites
- ~60% high-risk patients
- High-risk defined as nicotine use, BMI ≥ 30 and/or Type 2 diabetes
Rigorous fusion assessment criteria
- Thin-cut CT
- Multiple independent reviewers
†
High-risk includes nicotine use, BMI ≥ 30 and/or Type 2 diabetes.
Thoughtfully designed for ease of use and efficiency in the operating room.1,2
Moldable and designed for surgical site retention1,2
Handling
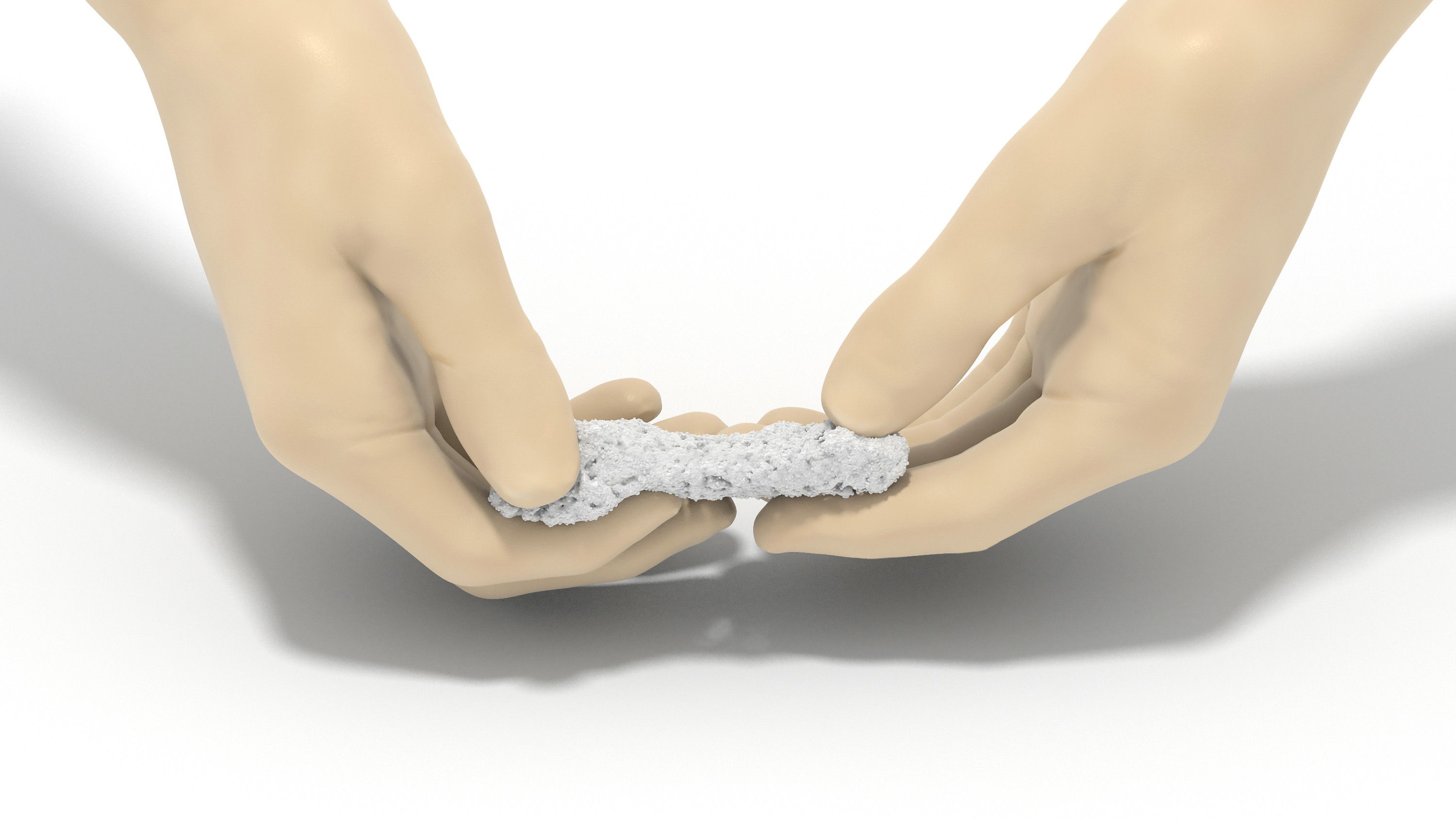
PearlMatrix contains a fibrous and moldable collagen matrix carrier purpose-built for optimized handling and surgical site retention.1,2
- Fibrous, cohesive, and moldable
- Naturally resists migration
- Allows for precise implantation
- Conforms to maximize bone-to-graft contact
Packaging and storage

PearlMatrix is conveniently packaged as a freeze-dried material that does not require freezing or thawing.1,2
- Stored at room temperature
- Three year shelf life
- Provided sterile and intended for single use only
Preparation
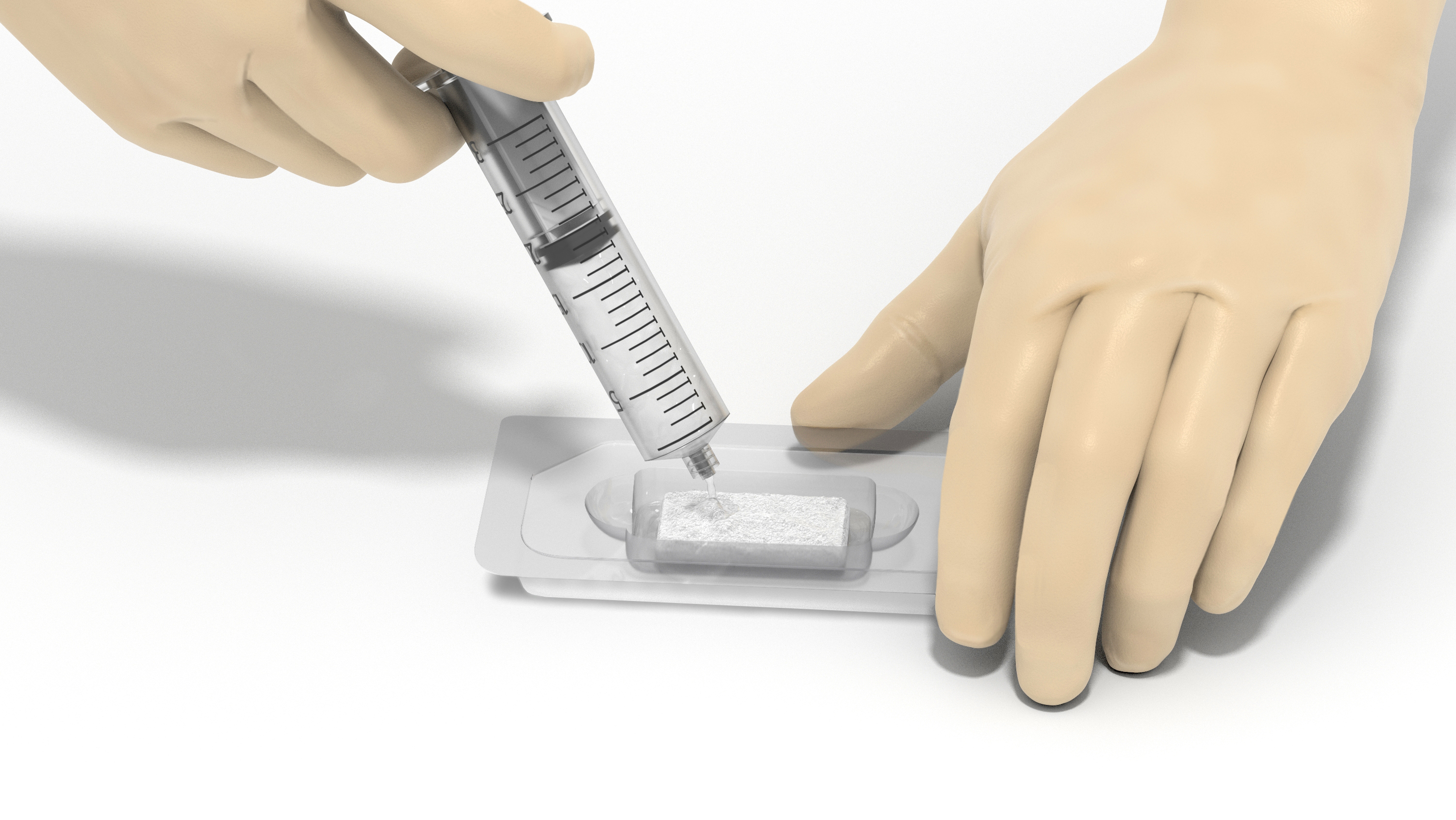
PearlMatrix is quickly hydrated with a simple preparation technique.1,2
- Quickly hydrated with sterile surgical solution (ie, saline or Ringer's lactate)
- Conveniently packaged in a sterile hydration tray
- No activation time or waiting for binding
Your patients can’t wait.
Neither should you.
Description | Catalog # |
|---|---|
PearlMatrix Bone Graft, 1.0cc | 730-010 |
PearlMatrix Bone Graft, 2.5cc | 730-025 |
PearlMatrix Bone Graft, 5.0cc | 730-050 |
PearlMatrix Bone Graft, 10.0cc | 730-100 |
Indications and Important Safety Information for PearlMatrix™ Peptide Enhanced Bone Graft (Rx Only)
Important Safety Information
PearlMatrix™ Bone Graft is indicated for intervertebral body fusion of the spine in skeletally mature patients. PearlMatrix™ Bone Graft is intended to be used in conjunction with a PEEK, titanium alloy, or PEEK/titanium interbody fusion device and supplemental internal spinal fixation systems cleared by the FDA for use in the lumbosacral spine. The system is to be used in patients who have had at least six months of non-operative treatment. PearlMatrix™ Bone Graft is intended for use at one level in the lumbar spine (L2-S1) for the treatment of degenerative disc disease (DDD) with up to Grade I spondylolisthesis. DDD is defined as back and/or radicular pain of discogenic origin with degeneration of the disc confirmed by history, physical exam, and radiographic studies.
The following open or minimally invasive surgical approaches may be used with PearlMatrix Bone Graft:
- ALIF (anterior lumbar interbody fusion)
- TLIF (transforaminal lumbar interbody fusion)
- PLIF (posterior lumbar interbody fusion)
- OLIF/ATP (oblique lumbar interbody fusion/anterior to psoas)
- LLIF (lateral lumbar interbody fusion)
PearlMatrix should not be used in situations where there is an absence of load-bearing structural support at the graft site, sensitivity to components or the product, active infection at the operative site, or operative site subject to excessive impact or stress.
Care should be exercised in treating individuals with preexisting conditions that may affect the success of the surgical procedure such as individuals with bleeding disorders of any etiology, long-term steroidal therapy, immunosuppressive therapy or high dosage radiation therapy. The effect of PearlMatrix on pregnant or nursing patients has not been evaluated. PearlMatrix in a TLIF procedure was associated with a higher rate of secondary surgical interventions compared to local autograft.
PearlMatrix should only be used by physicians who are experienced with lumbar interbody fusion procedures and in surgical procedures where it can be adequately contained at the bony void or defect.
To learn more about PearlMatrix, its indications, contraindications, warnings, precautions and potential adverse events, visit our website at www.cerapedics.com or refer to the PearlMatrix Instructions for Use for complete safety and risk information.
References:
- PearlMatrix US Instructions for Use. Cerapedics.
- Cerapedics. Data on file. September 9, 2024.
- Bono CM, et al. Spine. 2007;32(4):417-422.
- Mariscal S, et al. In preparation.
- Abjornson C, et al. Int J Spine Surg. 2018;12(6):757-771.
- Nguyen H, et al. Biochem Biophys Res Commun. 2003;311(1):179-186.
- Yang XB, et al. Tissue Eng. 2004;10(7-8):1148-1159.
- Liu Q, et al. J Orthop Res. 2012;10:1526.
- Thorwarth M, et al. Biomaterials. 2005;26(28):5648-5657.
- Lindley EM, et al. J Biomed Mater Res B Appl Biomater. 2010;94(2):463-468.